
Source of the article: Molecular Manor
AuthorMaster Mu Zi
Polyacrylamide gel electrophoresis (PAGE) is a commonly used electrophoresis technique that uses polyacrylamide gel as the supporting medium and can be used for the separation of proteins and nucleic acids.

I. Polyacrylamide gel
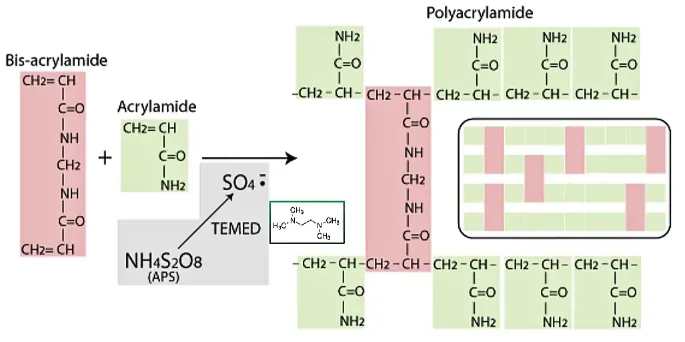
Polyacrylamide gel is formed by the polymerization of monomers acrylamide (Acr) and N,N '-methylene Bis acrylamide (Bis) under the action of initiators (eg.APS) and accelerators (eg.TEMED).
1. Aggregation principle
During the aggregation processThe free hydroxyl group of tetramethylethylenediamine (TEMED) promotesThe aqueous solution of ammonium persulfate [(NH₄)₂S₂O₈] generates SO4²⁻ radicals, which cause monomersThe C-C double bond of acrylamide and N,N '-methylene diacrylamide is opened and activated, an activated free radicalAcrylamideIt can occur between them.Polymerization forms long chains of acrylamide.Simultaneously activated methylene diacrylamide forms methylene bond cross-links between the continuously elongated acrylamide chains, thereby creating a cross-linked three-dimensional network structure.
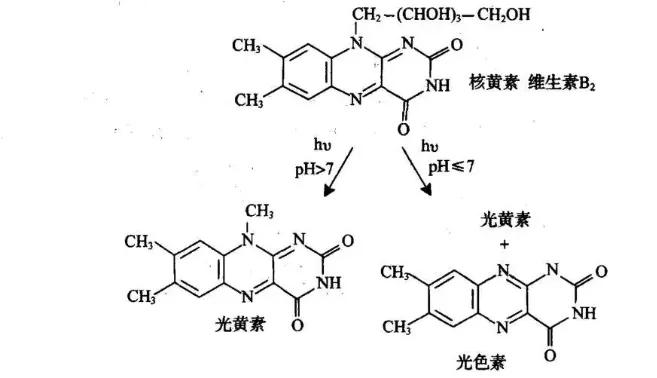
Acrylamide also has a photopolymerization method, with riboflavin as the catalyst. Riboflavin can generate free radicals under light, catalyzing the polymerization reaction.
2. ThickDegree control
The strength, elasticity, transparency, viscosity and pore size of the polymerized polyacrylamide gel all depend on the concentration of the gel and the degree of crosslinking.
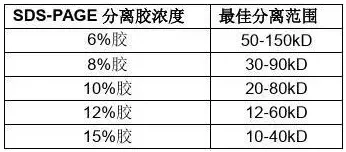
The pore size of polyacrylamide gel can be controlled by changing the concentrations of acrylamide and methylene diacrylamide, with the concentration of acrylamide ranging from 3% to 30%. Low-concentration gels have larger pore diameters. For instance, 3% polyacrylamide gel has no significant obstructive effect on proteins and can be used as a concentrated gel for plate isoelectric focusing or SDS-polyacrylamide gel electrophoresis, as well as for DNA separation. High-concentration gels have smaller pore sizes and act as molecular sieves for proteins. They can be used in electrophoresis where proteins are separated based on their molecular weights. For instance, gels with a pore size of 10% to 20% are often employed as separation gels in SDS-polyacrylamide gel electrophoresis.
3. Advantages
Polyacrylamide gel has been widely applied due to its many outstanding advantages. Its main advantages are:
1) The concentration of the gel and the degree of crosslinking can be controlled at will, thereby obtaining different effective pore sizes, which can be used to separate biological macromolecules of different molecular weights.
2) It can combine the molecular sieve effect and the charge effect in the same method to achieve higher sensitivity. Moreover, the polyacrylamide gel is an amide polymer bonded by C-C- bonds, with only the inactive amide group -CO-NH2 on the side chains and no other charged ionic groups. It has good chemical inertness and will not cause "electroosmotic" during electrophoresis.

3) Because high-purity monomer raw materials can be produced, the repeatability of electrophoretic separation is good; Moreover, the gel has good mechanical strength, elasticity, is not easy to break, is convenient for operation and storage, and can also be used as an inert carrier for immobilized enzymes.
4) The gel has high transparency, which is convenient for photographyIt can be copied without ultraviolet absorption and without staining, and can be used for gel scanning at ultraviolet wavelengths for quantitative analysis.
Ii. Classification of Polyacrylamide Gel Electrophoresis
Continuous and discontinuous electrophoresis systems
PAGE is classified into two major categories based on whether it has a concentration effect: continuous systems and discontinuous systems.
1) A continuous system refers to a system where the pH value of the buffer and the pore size (concentration) of the gel are the same throughout the electrophoresis system. Under the action of an electric field, charged particles mainly rely on charge and molecular sieve effects, and are mainly used for analyzing nucleic acid samples.
2) The discontinuous system is composed of electrode buffer, concentrated gel and separation gel: ① The concentrated gel is a macroporous gel with a 4% acrylamide concentration, and the gel preparation buffer is Tris-HCl with a pH of 6.8; ② The separation gel is a small pore gel with a concentration of 12.5% acrylamide, and the gel preparation buffer solution is Tris-HCl with a pH of 8.8. ③ The electrode buffer is Tris-glycine buffer with a pH of 8.3.
Two types of gel pore sizes, two buffer systems and three pH values make the discontinuous system form discontinuations in gel pore size, pH value and ionic composition of the buffer solution. The charged particles moving in the electric field not only have charge effect and molecular sieve effect, but also have concentration effect. Therefore, the clarity and resolution of the separation bands are better than those of the continuous system, and it is mainly used for the separation of protein samples.
2. Denatured and non-denatured electrophoresis systems
PAGE has two forms: Native-PAGE and denatured polyacrylamide gel electrophoresis.

1) Nondenatured polyacrylamide gel electrophoresis (Native-PAGE
Native-PAGE can enable biological macromolecules to maintain their natural shape and charge during electrophoresis. Their separation is based on the differences in their electrophoretic mobility and the molecular sieve effect of the gel. Especially after electrophoretic separation, it can still maintain the biological activity of biological macromolecules such as proteins and enzymes, which is of great significance for the identification of biological macromolecules.
In the non-denatured polyacrylamide gel electrophoresis of proteins, they are gradually separated in a gradient based on the molecular weight of the protein, its shape, and the amount of charge it carries. In the non-denatured polyacrylamide gel electrophoresis of DNA, DNA moves in a double-stranded state, and its mobility is affected by the base composition and sequence.
2) Denatured polyacrylamide gel electrophoresis
In denatured polyacrylamide gel electrophoresis, denaturants are added. The separation of biological macromolecules is only based on molecular weight. Protein denaturants are often SDS, and nucleic acid denaturants are often urea, formamide, etc.
SDS-PAGE is the most commonly used protein expression analysis technique. The principle of this technique is to separate proteins in the electrophoresis gel based on their different molecular weights in the sample. It is usually used to detect the expression of proteins and analyze the purity of the target protein, etc.
The experimental process of SDS-PAGE
1) Sample processing
Proteins contain many amino (+) and carboxyl (-) groups. Different proteins exhibit different charges at different pH values. To ensure that the mobility of proteins in electrophoresis is only related to their molecular weight, some treatments are usually carried out before loading. That is, add the upper buffer solution containing SDS and β -mercaptoethanol/DTT to the sample.
Depending on the different purposes of sample separation, there are mainly three treatment methods: reducing SDS treatment, non-reducing SDS treatment, and reducing SDS treatment with alkylation.
① Restore the SDS treatment
After adding SDS and DTT/β -mercaptoethanol to the sample loading buffer, the protein conformation is dissociated and the charge is neutralized, forming a molecule where SDS binds to the protein. In electrophoresis, only the molecular weight is consideredSeparation.
Bromophenol blue dye is usually added to the sample loading buffer to monitor the electrophoresis process. Additionally, an appropriate amount of sucrose or glycerol can be added to increase the density of the solution, allowing the sample solution to sink to the bottom of the sample groove during loading.

Generally, electrophoresis is handled in this way: dilute the sample to an appropriate concentration, add the loading Buffer, centrifuge, boil in boiling water for 5 minutes, and then centrifuge and add the sample.
② Reduced SDS treatment with alkylation
The alkylation effect of amine iodoacetate can well and firmly protect the SH group, keeping the completely denatured protein in a reduced state. In addition, iodoacetate can capture excessive DTT and prevent the texture phenomenon during silver dyeing. Add 10μL of 20% iodoacetate to 100μL of sample buffer and incubate at room temperature for 30 minutes.
③ Non-reducing SDS treatment
Samples such as physiological body fluids, serum, and urine are generally boiled in 1%SDS water for 3 minutes without adding a reducing agent. As a result, the protein folding is not disrupted and they are not used for molecular weight determination.
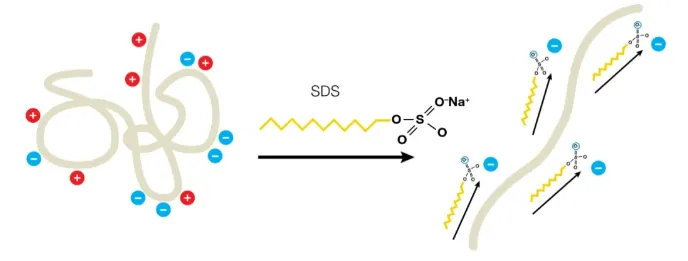
SDS (sodium dodecyl sulfate) is an anionic detergent that can break hydrogen bonds within and between molecules and destroy the secondary and tertiary structures of protein molecules. The strong reducing agent β -mercaptoethanol/dithiothreitol can break the disulfide bonds between cysteine residues.
After adding the reducing agent and SDS to the sample and gel, the protein molecules are completely denaturing and depolymerizing into polypeptide chains. The SDS combines with the amino acid side chains on the polypeptide chains to form protein-SDS micelles (on average, one SDS molecule is bound to every two amino acid residues), and they carry a large amount of negative charge, which far exceeds the original charge of the protein. This eliminates the charge differences between different molecules.
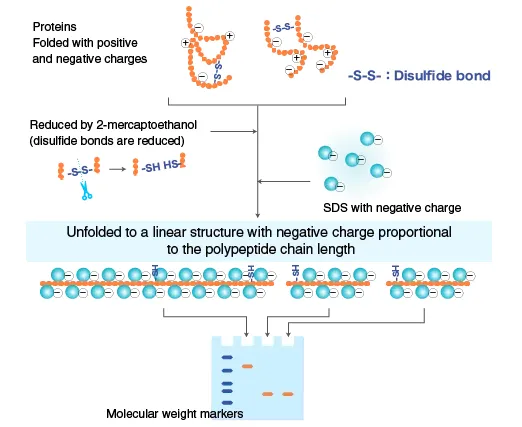
After SDS binds to proteins, it can also cause conformational changes. The protein-SDS complex forms a long elliptical rod similar to a "cigar" shape. The short axis lengths of the SDS complexes of different proteins are all the same, approximately 18A (1A =0.1nm). The mobility of such protein-SDS complexes in the gel It is no longer affected by the charge and shape of proteogen, but only depends on the size of the molecular weight.
2) Gel configuration

When preparing gels, the first step is to select an appropriate gel concentration based on the conditions of the samples to be separated. If larger proteins are to be separated, gels with lower concentrations such as 10% or 7.5% (for larger pore sizes) should be used. For the separation of smaller proteins, using a higher concentration gel (with smaller pore size) can achieve better separation results.
After the separation gel polymerizes, a layer of concentrated gel (about 1 cm) is usually added on top, and a sample comb is inserted into the concentrated gel to form a sample loading groove.
Concentrated glue mainly serves to pile up. The gel concentration is relatively low (4%), and the pore size is large. Various proteins can pass freely without being hindered by the gel pore size. When the sample is added to the concentrated gel, it is concentrated into a narrow zone through the migration effect of the large-pore gel.
3) Electrophoresis process
During the electrophoresis process, the sample first passes through the concentrated gel and is concentrated due to the isokinetic electrophoresis phenomenon before entering the separation gel. This is because there are mainly three anions in the electrophoresis buffer, namely Cl⁻, the glycine anion and the negatively charged protein-SDS complex. At the pH value of the concentrated gel, only a small amount of glycine is ionized, so its electrophoresis mobility is the smallest, while the electrophoresis mobility of Cl⁻ is the largest. Under the influence of an electric field, the initial migration speed of Cl⁻ is the fastest. Thus, a low-ion concentration region, namely a low-conductivity area, is formed behind Cl⁻. The low-conductivity area generates a higher electric field intensity, so the ions behind Cl⁻ will accelerate their movement under the action of the higher electric field intensity. After reaching a stable state, a stable and moving interface is formed between Cl- and glycine. The protein-SDS complex, due to its relatively small amount, accumulates near the interface between glycine and Cl⁻ and is concentrated into a very narrow band (which can be concentrated three hundred times).

When glycine reaches the separation gel, due to the high pH value of the separation gel (usually pH8.8), the degree of glycine dissociation increases, and the electrophoretic migration speed exceeds that of the protein-SDS complex. The interface between glycine and Cl⁻ soon exceeds that of the protein-SDS complex. At this point, the protein-SDS complex undergoes electrophoresis in the separation gel at its own electrophoretic migration speed and moves towards the positive electrode. Because the protein-SDS complexes carry equal charges per unit length, they migrate from the concentrated gel to the separating gel at the same speed. After entering the separating gel, due to the molecular sieve effect of polyacrylamide, small molecule proteins can easily pass through the gel pores with low resistance and fast migration speed. Macromolecular proteins, on the other hand, encounter greater resistance and are lagged behind. As a result, during electrophoresis, proteins are separated based on the size of their respective molecular weights.
4) Gel staining analysis
Bromophenol blue indicator is a smaller molecule that can freely pass through the gel pore size, so it shows the leading position of electrophoresis.
When the indicator reaches the bottom of the gel, stop the electrophoresis and remove the gel from the plate.Stain for several hours in an appropriate staining solution (such as the commonly used Coomassie brilliant blue), and then decolorize overnight. The decolorizing solution removes the background dye that has not bound to the protein in the gel. At this point, the stained protein bands in the gel can be clearly observed.

2. Characteristics of high pH alkaline discontinuous systems
The pH of the concentrated gel (6.8) is about 3 pH lower than the pKa of the slow ion glycine (9.8), so α≈0.001. That is, in the concentrated gel, only 0.1% of glycine dissociates, so it migrates very slowly in the electric field and is a slow ion.
2) The fast ion Cl⁻, due to its almost complete dissociation, has the highest electrophoretic mobility and is not affected by pH changes.
3) The pH of the separation gel (8.8) is about one pH lower than the pKa of the slow ion, with α≈0.1. Therefore, when the glycine dissociation in the separation gel increases, the electrophoretic mobility increases, and it moves to the front of the protein band.
4) The pKa of the conjugate base Tris in the electrode buffer solution is approximately 1 pH lower than that of the separation gel (8.8), which increases its buffering capacity. The pH of the electrode buffer solution is 8.3, which is close to the pKa of Tris (8.1), in order to achieve the maximum buffering capacity.
3. Application of SDS-PAGE
1) Determination of the molecular weight of unknown proteins
When the molecular weight is between 15KD and 200KD, the mobility of the protein has a linear relationship with the logarithm of the molecular weight, which conforms to the following formula: logMW=K-bX, where: MW represents the molecular weight, X represents the mobility, and both k and b are constants. If the mobility of a standard protein with a known molecular weight is plotted against the logarithm of the molecular weight, a standard curve can be obtained. For an unknown protein undergoing electrophoresis under the same conditions, its molecular weight can be calculated on the standard curve based on its electrophoretic mobility.

2) Detection of purity during the protein purification process
Purified proteins usually should have only one band on SDS electrophoresis, but if the protein is composed of different subunits, it may form several bands corresponding to each subunit during electrophoresis. Sds-polyacrylamide gel electrophoresis has a high sensitivity. Generally, it only requires proteins at the microgram level. Moreover, through electrophoresis, information about molecular weight can be obtained simultaneously. This information is very important for understanding unknown proteins and designing the purification process.
4. Common Questions about SDS-PAGE electrophoresis
1) Regarding the preparation of gel
The gel usually sets within 30 minutes to 1 hour. If it coagulates too slowly, it might be due to insufficient doses of TEMED and AP. If it solidifies too quickly, it might be due to excessive amounts of AP and TEMED. After the gel has solidified at room temperature, it can be left at room temperature for a period of time before use. It is not advisable to prepare and use it immediately or store it in the refrigerator. The former may lead to incomplete solidification, while the latter may cause SDS crystallization.
When the temperature is relatively low (in winter), the high-concentration gel is uneven, and wavy patterns are prone to appear at the lower part of the separated gel. At this point, the amounts of TEMED and peroxymonosulfate can be increased to accelerate their coagulation rate. High-concentration gels are brittle and prone to breakage. Under conditions of higher room temperature, the amounts of TEMED and peroxymonosulfate can be appropriately reduced.
2) Abnormal conditions of the strip
① The appearance of a "smiling" band (with both sides raised and the middle concave ︶) : in thicker gels, due to uneven cooling of the gel, the middle part does not solidify well.
② The appearance of a "frowning" shaped band (with both sides downward and the middle bulging ︵) : the general cause is that the air bubbles in the bottom gap between the two plates have not been completely removed. An appropriate amount of buffer solution can be added between the two plates to remove the air bubbles.
③ Tailing occurs: It is caused by poor sample dissolution or excessive concentration of the separation glue. Additionally, it could be due to excessive separation glue during the glue filling process, resulting in very little concentrated glue and failing to achieve the concentration effect, thus not pressing the sample into a single line. It is recommended to select an appropriate sample buffer and add an appropriate amount of sample solubilizer. If the electrophoresis buffer has been in use for too long, prepare it again. Reduce the gel concentration.
④ Texture phenomenon: It is mainly caused by insoluble particles in the sample. It is recommended to add an appropriate amount of sample solvent.
⑤ The bands in electrophoresis are too thick: The protein sample was not concentrated properly. Increase the length of the concentrating gel appropriately. Ensure the pH of the concentrated gel storage solution is correct (6.8); Reduce the voltage appropriately.
⑥ The Appearance and Handling of "Ghost Bands" : "Ghost bands" refer to the appearance of unknown bands at the top of the lanes or the formation of precipitates at the bottom of the sample loading Wells when running complex conformed protein macromolecules. This is mainly due to the oxidation and inactivation of the reducing agent during heating, causing the dissociated protein molecules to refold and recombine subunits. Since their molecular weights are usually larger than those of the target bands, unknown bands or precipitates are formed. It is recommended to add an appropriate amount of DTT or β -mercaptoethanol after heating and boiling to replenish the insufficient reducing agent, or an appropriate amount of EDTA can be added to prevent the oxidation of the reducing agent.
⑦ Blurred bands of the target protein: Improper selection of electrophoresis gel concentration (Tricine gel should be used for small molecule proteins below 10Kd); When hydrolyzing protein samples, pay attention to removing the endogenous hydrolase in the protein samples. Do not repeatedly freeze and thaw. The buffer solution and SDS should be freshly prepared. To avoid excessive sample addition and enhance resolution, the sample volume should be flexibly controlled based on the sample concentration and gel thickness. Generally, the sample loading volume is 10-15 μ l (i.e., 2-10 μ g of protein).
The protein samples that have been denatured by heating should be placed at room temperature for electrophoresis. They should not be left for too long and must not be stored in the refrigerator. This is because the disulfide bonds of proteins may break at high temperatures, but when exposed to air and the temperature drops, they will re-form. Moreover, during the prolonged storage process, the proteins may fold again.
3) Abnormal conditions of electrophoresis
① The electrophoresis voltage is very high but the current is very low (the voltage is above 50v, but the current is below 5mA) : The main reason is that the electrophoresis tank is not correctly assembled, and the current has not formed a path. This includes installing the inner and outer tanks in reverse, or having too little liquid in the outer tank, etc.
② The electrophoresis time is longer than normal: This might be due to the incorrect PH selection of the gel buffer system and the electrode buffer system, that is, the PH of the buffer system and the isoelectric point of the separated substance differ too little, or the ionic strength of the buffer system is too high.
③ Bromophenol blue does not serve as an indicator: In experiments, it sometimes occurs that bromophenol blue has escaped from the bottom of the plate, but the protein has not yet come down. The main reason might be that the concentrations of the buffer solution and the separation gel are too high. It is recommended to replace the buffer solution with the correct pH value and reduce the concentration of the separation gel.
④ After electrophoresis, there are many long streaks of impurities on the gel: It is recommended that the electrophoresis buffer solution not be recycled and the solution used to prepare the gel must be pure.
The influence of the gel mixing buffer system in electrophoresis:
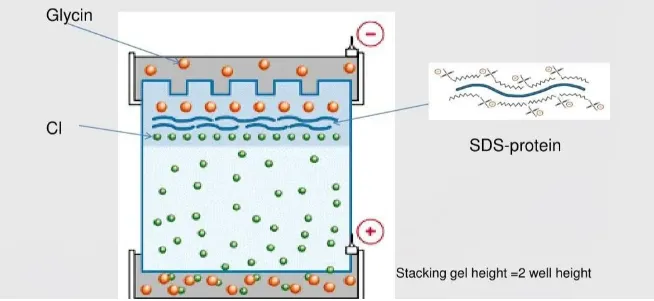
The main function of the buffer solution in the electrophoresis process is to maintain an appropriate pH. During electrophoresis, both the positive electrode and the negative electrode undergo electrolytic reactions. Oxidation occurs at the positive electrode, while reduction occurs at the negative electrode. Prolonged electrophoresis will cause the positive electrode to become acidic and the negative electrode to become alkaline. The buffer solution can maintain the pH of the two electrodes of the solution basically unchanged. In concentrated gel, the pH environment is weakly acidic, so the glycine dissociation is very little and the swimming efficiency is low. However, Cl⁻ is very high, and a band with lower conductivity is formed between the two. Protein molecules move between them and gather together, condensing into a narrow band. Therefore, the influence of pH on the entire reaction system is of vital importance. In experiments, if the problem still cannot be well solved after excluding other factors, this factor should be given primary consideration.
Iv. PAGE and DNA Analysis
1. Reagent preparation
①30% Acr-Bis (29:1) : 29g acrylamide, 1gN,N´ -methylene diacrylamide, add distilled water to make up to 100mL.
A 30% acrylamide aqueous solution can be stored at 4℃ for one month. During the storage period, acrylamide will hydrolyze into acrylic acid, increasing the electro-osmotic phenomenon during electrophoresis and slowing down the electrophoresis mobility. Acrylamide and methyldiacrylamide are reagents that are toxic to the central nervous system. Direct contact with the skin should be avoided during operation, but they are non-toxic after polymerization.
②10% ammonium persulfate (Aps) : 0.4g ammonium persulfate, 4mL distilled water.
③1×TBE buffer: 89mol/L Tris-borate, 2mol/L EDTA(pH8.0).
④ Sample buffer solution: 0.25% bromophenol blue, 0.25% xylene blue FF, 40% sucrose water solution (W/V), store at 4℃.
2. Gel preparation
Component name | Preparation method of 10% PAGE Gel (40ml) | 30% Acr-Bis (29:1) and TEMED should be stored at 4 ° C away from light, while the remaining components can be stored at room temperature. 2. Ammonium persulfate (APS) is a solid powder. Before use, it should be prepared into a 10% APS solution (0.1g APS plus 1mL double-distilled water). APS solution is best prepared and used immediately. Generally, it can be stored for one week at 4℃ and for half a year at -20℃. 3. TBE has a strong buffering capacity, is suitable for long-term electrophoresis, and has a relatively high resolution. 4. The coagulation rate of PAGE gel is closely related to temperature and the dosage of APS and TEMED. The polymerization rate of PAGE gel can be controlled by changing the dosage of APS and TEMED. 5. TEMED must be added last when making glue. |
30% Acr-Bis(29:1) | 13.3ml | |
Ammonium persulfate (APS, 10%) | 400ul | |
Tetramethylethylenediamine (TEMED | 20ul | |
dd H20 | 18.4ml | |
5X TBE electrophoresis buffer | 8ml |
3. Effective DNA separation range
Polyacrylamide [%(W/V)] | Effective separation range (bp | Xylene green FF | Bromophenol blue |
3.5 | 1000-2000. | 460 | 100 |
5 | 80-500. | 260 | 65 |
8 | 60-400. | 160 | 45 |
twelve | 40-200. | 70 | 20 |
15 | 25-150. | 60 | 15 |
20 | 6-100 | 45 | twelve |
4. VS agarose gel
Compared with agarose gel, polyacrylamide gel is more difficult to prepare and process, and has a narrower separation range. However, they also have outstanding advantages. Due to the discontinuous pH gradient, the sample is compressed into a narrow band, thereby enhancing the separation effect and improving the electrophoresis resolution, especially for the analysis of small DNA fragments (5 to 500bp).Within this range, DNA molecules that are only 1bp apart can also be clearly separated.
Secondly, the purity of the recovered DNA is very high and it can be directly used for microinjection of mouse embryos. This gel is also commonly used for the purification of primers, also known as PAGE purification.
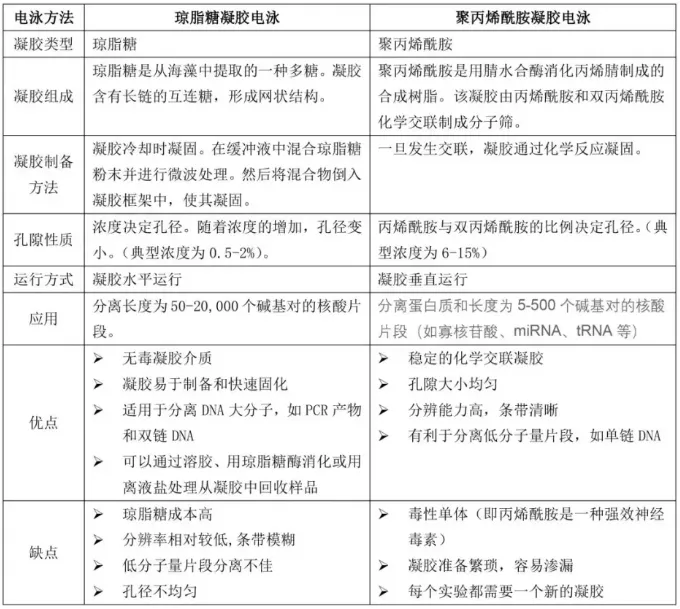
| vs. | PAGE | Agarose gel |
"Prepare" | Cumbersome and complicated | Simple |
Aperture | small | big |
Separation range | It can only separate nucleic acids with smaller molecular weights, but the resolution is high. | It can separate nucleic acids with relatively large molecular weights and has a wide separation range. |
Gel thickness | ≥0.2mm | ≥3mm |
Sample loading capacity | The resolution of large DNA (e.g., up to 10 micrograms) is not significantly affected. | small |
Direction of progress | Vertical | "Level" |
"Progress time" | long | short |
Cost | Higher | Cheap |
Safety | Non-toxic | Toxic |
5. DNA staining
Unlike agarose gel, nucleic acid dyes cannot be added when filling polyacrylamide gel, as dyes can affect the polymerization of acrylamide, hinder the migration of DNA and deform DNA bands. So, generally, staining is carried out by soaking after electrophoresis.
6. DNA recovery
The gelation principles of PAGE and agarose are different: The coagulation of polyacrylamide gel is a chemical process, in which the acrylamide monomer polymerizes into a high polymer through a chemical reaction. The characteristic of a chemical reaction is that the higher the temperature, the faster the reaction rate. The gelation of agarose gel is a physical process. The long chains of agarose remain unchanged throughout the process; what changes is the combination of secondary bonds such as hydrogen bonds. The lower the temperature, the stronger the hydrogen bond bond.
Therefore, DNA is generally recovered from polyacrylamide gel through crushing and soaking methods, as the gel does not melt at the normal recovery temperature and only returns to the solution through the spontaneous movement of DNA from the gel.
7. Precautions for electrophoresis
1) General use0.5X/1X TBE, 1-8V /cm low-voltage electrophoresis. If performed at a higher voltage, the heat generation in the middle of the gel will be uneven, causing DNA bands to bend and even leading to the cleavage of small DNA fragments.
2) The thickness of the spacer glass plate can be 0.5 to 2mm. The thicker the gel, the more heat is generated during electrophoresis. Excessive calories can cause the "smiling" phenomenon of DNA bands.
3) The same batch of electrophoresis buffer must be used in the buffer tank and gel. Even minor differences in ionic strength or PH can cause dysfunction of the buffer, thereby severely distorting the migration of DNA fragments. To prevent band tailing after electrophoresis, the strength of salt ions in the sample should be reduced as much as possible.

0756-8699969
Address: No. 88, Shuian 1st Road, Nanping Science and Technology Park, Xiangzhou District, Zhuhai City, Guangdong Province
Email: marketing@biori.com
Wechat official account |
Product consultation |
Join us |
Video Account |






