
Article source: IVD Practitioner Network
Author: Suo Yan
Reference materials: Clinical Laboratory Medicine, Thermo Fisher Life Science Service Platform, Laboratory Vision Network, etc
The most intuitive way to judge the results of fluorescence quantitative PCR is to check whether the amplification curve is normal. Many teachers are troubled by abnormal amplification curves. Next, we will take a look at the analysis of common abnormal amplification curves together with several examples.
1. Normal PCR amplification curve
As the experiment progresses, the amplification products accumulate continuously, and the corresponding fluorescence signals also increase. A fluorescence signal is collected for each cycle. After a certain number of cycles (for example, 40 cycles), the following amplification curve graph can be obtained (the horizontal axis represents the number of amplification cycles, and the vertical axis represents the intensity of fluorescence).

Among them, it is also necessary to clarify some basic concepts, such as:
Baseline: It refers to the period during which the fluorescence signal changes little in the first few cycles of the PCR amplification reaction. Close to a straight line, which is called the baseline. Just understand this. It has no impact on the experimental operation.
Threshold line: Generally speaking, the fluorescence signals of the first 15 cycles are used as the fluorescence background signals. The threshold is set at 10 times the standard deviation of the fluorescence signals from 3 to 15 cycles during the exponential phase of PCR amplification (as indicated by the arrow in the figure).
It sounds so serious, but in fact, the threshold lines automatically set by many instruments can basically meet our experimental requirements and there is no need for us to set them manually.
CT value: We often use the CT value to calculate our relative content, that is, the number of amplification cycles experienced when the fluorescence signal of the amplification product reaches the set threshold line, which is the value shown on the horizontal coordinate at the intersection of the amplification curve and the threshold line in the figure.
2. Standard curve
What's the use of making a standard curve? The standard curve can be used to determine the initial quantity of an unknown sample. If absolute quantification is required, a standard curve must be available.
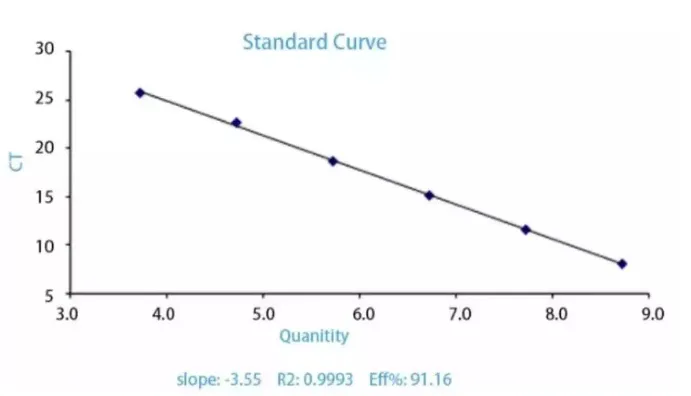
How to do it? The CT value of each template has a linear relationship with the logarithm of the initial copy number of that template. The larger the initial copy number, the smaller the CT value. The standard substance is diluted at a certain multiple for about 5 to 6 concentrations and the reaction is carried out according to the reaction conditions of RT-PCR. Plot a linear curve with the logarithm of the copy number of the standard substance as the abscissa and the CT value as the ordinate. The copy number of the unknown sample can be calculated based on the curve equation.
So, the question arises: How is the reference material for this standard curve determined?
(1) If it is for absolute quantification, the requirements for the standard substances are relatively high, and the preparation process is also more complex, but the results are more accurate.
Requirement
It must be accurately quantified, standardized and stable plasmid DNA (or genomic DNA, purified PCR products, etc.)
5 to 6 dilution gradients;
The PCR reaction instruments should be of the same type and operated simultaneously.
The reaction conditions are consistent: reaction system, parameters, etc.
Anyway, as long as everything that can be agreed upon is consistent, that's fine.
(2) If we just want to check the amplification efficiency (E) (E=10-1/ slope) and verify whether the PCR reaction system meets the requirements, sometimes we also use the sample to be tested as the standard, gradually dilute it and then proceed with the reaction. Finally, we observe whether the slope of the standard curve and R2 meet the requirements. I'm rather lazy and often use this method. However, some articles have relatively high requirements, so you still have to do it obediently.
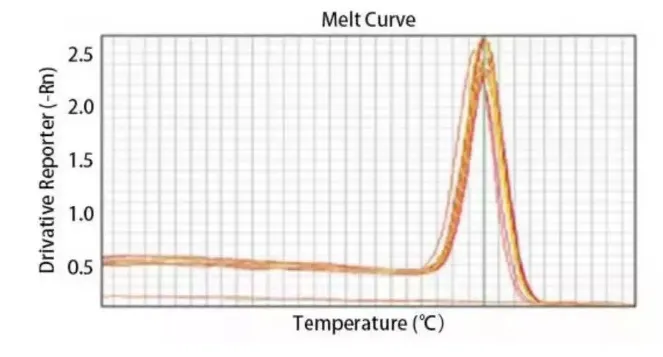
3. Melting curve
In simple terms, it is to examine the specificity of the results of the dye labeling method. To put it more simply, it's about determining in no time whether your primers are effective.
I won't explain the complex principles. The key lies in how to view and how to judge. If the reaction product is single, a sharp peak will appear on the curve. If there is dimer or non-specific amplification, at least two peaks or even worse will occur. When using SYBR Green dye, it is necessary to make a dissolution curve, after all, this is a non-specific dye.
In the experiment, people will still encounter many problems. Here are a few of the more common ones:
Does the amplification curve have no plateau period after 40 cycles? Or does the amplification curve simply fail to show an exponential increase?
In this case, it's very likely that your initial template concentration is too low. Even after 40 cycles, it won't reach the plateau period. You can verify whether this is the problem by increasing the number of cycles.
(2) Issues with the melting curve: For instance, multiple peaks, or a tiny peak in front of a sharp peak, or no peak at all?
This is related to the primers and the temperature of the reaction system. If there is no peak or it is chaotic, it is very likely a problem with the primers. It is recommended to redesign the primers. Sometimes there are small peaks, which can be eliminated by optimizing the reaction conditions, such as the annealing temperature.
Analysis of Common abnormal curves
1. Confirm whether the software Settings are correct
Refer to the kit manual to check whether the time, temperature, cycle number, fluorescence collection and other Settings are correct. One setup issue that is often overlooked is that it is necessary to check whether the selected reagent contains ROX as the reference fluorescent dye.
2. Ensure that the consumables and instrument accessories are used correctly
Consumables are quite important for fluorescence quantitative PCR. Many abnormal amplification curves are caused by improper use of consumables. Common consumables related issues include:
1) Incorrect use of consumables corresponding to ordinary PCR instruments
Ordinary PCR consumables usually have poor light transmittance, which can cause abnormal fluorescence amplification curves, such as discounted amplification curves (Figure 3).


2) Consumables that match the specifications of the instrument's heating module were not selected
The heating modules of the fluorescence quantitative PCR instrument are available in two types: 0.2ml and 0.1ml. If the 0.1ml heating module is replaced with 0.2ml consumables, it may cause the consumables to be flattened and even lead to machine malfunctions. If the 0.2ml heating module is replaced by 0.1ml of consumables, it will cause the outer wall of the reaction tube and the temperature control module of the fluorescence quantitative PCR instrument to not have sufficient contact, resulting in insufficient heat conduction, which in turn affects the activity of the enzyme, leading to poor repeatability (Figure 4), or multiple peaks in the dissolution curve (non-specific amplification), or even false negative results.
3) Poor quality consumables were used
At present, there are numerous types of fluorescent quantitative PCR consumables on the market, and their quality varies greatly. When choosing consumables, it is advisable to select those with better quality and brand; otherwise, it may cause a series of problems and lead to unnecessary waste.
For instance, poor-quality consumables may cause unstable fluorescence values, which can lead to incorrect automatic baseline subtraction in the reaction Wells and result in inaccurate CT values (left image of Figure 5). After replacing with high-quality consumables, the fluorescence signal returns to normal (right image of Figure 5).

Figure 5: Unstable Fluorescence Values Caused by Poor-quality consumables (Part 1)
If the sealing film quality of the PCR reaction plate used is poor and it is affected by water vapor during the PCR process, it is prone to deformation (Figure 6), which will cause "optical warping", that is, the "optical distortion" phenomenon (left image of Figure 7). In this experiment, since ROX was used as the reference dye, ROX effectively corrected the variation of this fluorescence signal, and the results of the homogenized amplification graph were normal.

Figure 6: Water vapor deforms the sealing film during the PCR process

Figure 7: Optical distortion result graph and ROX homogenization result graph
In addition to consumables, it is also necessary to check whether the accessories used in conjunction with the instrument are used correctly. For example, when using the 8-tube bracket that comes with it, only the lower part of the bracket should be used. If the upper part is forgotten to be removed, it may affect amplification (Figure 8).

Figure 8: The upper part of the bracket was not removed, resulting in some targets not being amplified
3. Confirm whether the reagents used are normal
If all the Settings are correct and the consumables and accessories are fine, but the amplification curve is still abnormal, it is necessary to determine whether the reagents used are normal at this time.
First, it is necessary to determine whether the reagent is valid, including checking whether it is within the validity period, whether it has been frozen and thawed multiple times, and whether the transportation conditions are normal. The validity of the reagent can be determined by comparing the batch numbers of the reagents and combining them with the positive control results in the experiment. Secondly, it is necessary to observe the volume of the reagent after amplification. If there is evaporation of the reagent during the amplification process, it may cause the amplification curve to rise. If ROX is added as a reference fluorescence in the experimental system, the ROX fluorescence can distinguish whether the rise of the amplification curve is true amplification or evaporation.
For instance, if there is evaporation in the reaction system, water loss will occur, the ROX concentration will increase, and the ROX reference fluorescence will rise (left image of Figure 9), while the ROX fluorescence in the normal well will remain unchanged (right image of Figure 9). Therefore, before adding reagents and putting them on the machine, it is essential to check whether the consumables have been sealed to prevent reagent evaporation. Severe reagent evaporation may even lead to aerosol contamination throughout the laboratory.


Figure 9: The amplification curve rises due to reagent evaporation
4. Determine the operational details during the experiment
If all the above are normal, it is also necessary to determine the operational details during the experiment.
For instance, when preparing the standard curve, whether it is a gradient dilution standard substance. If it is not a gradient dilution standard substance, it may cause abnormal amplification efficiency or R2 value of the standard curve.
Check if the reagents taken out of the freezer are well mixed. If the reagents are not well mixed after melting, the reagents taken out at this time will not be uniform, which will affect the subsequent amplification.
After the quantitative PCR premix and nucleic acid template are added, it is necessary to check whether they are well mixed. If they are not well mixed, especially when the sample volume is relatively large (exceeding 20% of the PCR system), the optical mixing phenomenon is more likely to occur.
Because the aqueous phase of the sample is on the upper layer and the reagent (containing glycerol components) is on the lower layer, it is not fully mixed in the early PCR process, which will cause refraction and strong fluorescence. However, after several heating cycles, the sample and the premix will be well mixed and the fluorescence will become stable (Figure 10).
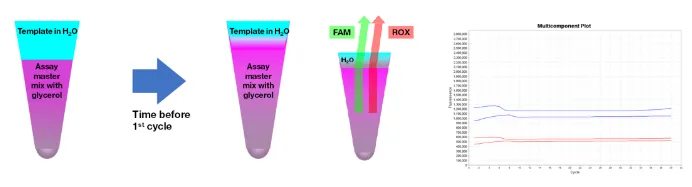
Figure 10: The phenomenon of the sample evaporation premix not being thoroughly mixed
Also, try to avoid large air bubbles in the reaction system on the machine. If there are air bubbles, refraction is likely to occur in the middle, which may interfere with the collection of fluorescence (Figure 11). Therefore, special attention should still be paid to the details of real-time fluorescence quantitative PCR experiments.
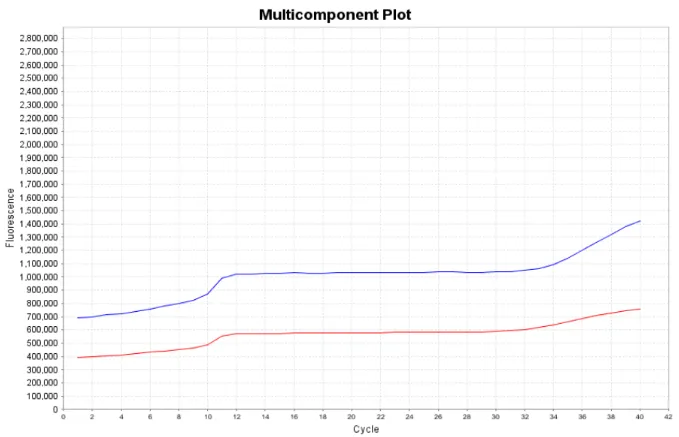
Figure 11: There are bubbles in the reaction system
Other abnormal curves, practical analysis:
Abnormal Curve 1: PCR amplification inhibition

Cause analysis: Amplification inhibition exists in H07
Solution: Dilute the sample and then amplify it (the influence of the inhibitor can be eliminated by diluting the sample)
Abnormal Curve 2: Automatic baseline setting issue

Cause analysis: Automatic baseline issue
Solution
Set the baseline manually. Set Baseline End to "the previous cycle before the amplification signal appears", that is, the original image exhibition was 26, set it to 20, and click OK to restore the normal curve.
Abnormal curve 3: No Ct value appeared in the results of the fluorescence quantitative PCR experiment

Cause analysis
(1) Incorrect PCR parameter Settings: Fluorescence signal acquisition was not set when designing the cycle parameters;
(2) Template degradation: Avoid the introduction of impurities during sample preparation and repeated freeze-thaw cycles;
(3) Primer or probe degradation: Its integrity can be detected by PAGE electrophoresis;
(4) The computer has been set to automatic sleep mode.
Solution: Conduct a thorough check one by one based on the cause analysis and handle the symptoms accordingly.
Abnormal curve 4: Oblique linear amplification curve

The first PCR test showed an oblique linear amplification curve. After re-testing, the results were basically all normal and negative.
Cause analysis
(1The liquid separation was inaccurate. After inspecting and testing the reaction tube, it could be found that the amount of PCR reaction liquid in the tube was significantly less than that in the normal tube.
(2The reaction tube has poor air tightness. During the reaction process, the evaporation of the solution causes an increase in the concentration of the probe, resulting in a slanted straight curve.
Solution
(1The liquid distribution is uniform. Before going on the machine, check whether the liquid levels in the eight-pipe connection are on the same horizontal line.
(2The eight-pipe cap should be tightly closed. Before going on the machine, check if there are any gaps.
Abnormal curve 5: Jumps from the end of the amplification curve

In the PCR experiment results, there is a phenomenon where the curve starts off at the end but does not complete amplification.
Cause analysis
(1) A jump from the end of the negative parameter curve usually indicates the presence of contamination.
(2) Re-examine the samples to rule out the possibility of non-specific amplification;
(3) Normal samples may also have a jump from the end of the curve, indicating a low sample concentration or inaccurate sample addition.
Solution
(1Rule out whether there is pollution or not;
(2) After ruling out the contamination cause, re-examine the sample. If the curve after re-examination is a negative straight line, it indicates that the previous tail jump was non-specific amplification. If the curve remains consistent with the previous one after re-examination, it indicates that the tail jump suggests that the concentration of the viral nucleic acid to be tested in the sample is low.
Abnormal Curve 6: Mountain Domain type curve

Cause analysis
Severe evaporation will show a curve of first rising and then falling, while mild evaporation will be a slow rise. These two types of abnormal curves can be verified by checking whether the liquid level in the reaction tube has dropped after detection.
Solution
Before the on-machine testing, check the tube caps of the eight-tube connection to ensure there are no gaps before amplification on the machine.
Abnormal curve 7: The amplification curve has either upward or downward peaks
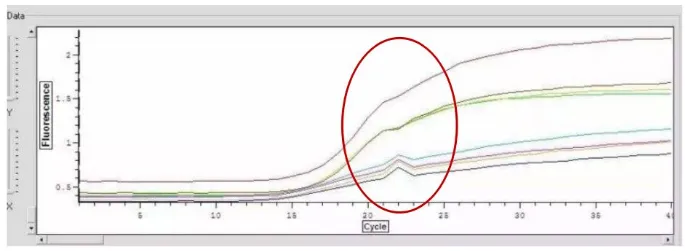
Cause analysis
(1) Abnormal amplification curve caused by unstable instrument (it may also be due to sudden power failure or unstable voltage);
(2) If the peak is downward, it might be due to the instability of the emission light source caused by the aging of the halogen lamp (referring to the exciter of the halogen tungsten lamp source).
(3) If a sharp peak appears in a single well position, in addition to considering the issue of the instrument or the well position, it is also necessary to consider whether the reaction system is caused by bubbles.
Solution
Check one by one according to the cause analysis and deal with the symptoms accordingly.
Background knowledge: Indicators for judging whether the amplification curve is good

1. The inflection points of the curve are clear, especially the exponential period of low-concentration samples is obvious. The overall parallelism of the amplification curve is good, with a flat baseline and no upward trend. The exponential period of the amplification curve of low-concentration samples is obvious.
2. The slope of the exponential phase of the curve is directly proportional to the amplification efficiency; the greater the slope, the higher the amplification efficiency.
3. The standard baseline is flat or slightly lowered, with no obvious upward trend.
4. The amplification curves of each tube have good parallelism, indicating that the amplification efficiencies of each reaction tube are similar.
0756-8699969
Address: No. 88, Shuian 1st Road, Nanping Science and Technology Park, Xiangzhou District, Zhuhai City, Guangdong Province
Email: marketing@biori.com
Wechat official account |
Product consultation |
Join us |
Video Account |






