

In recent years, mRNA vaccine technology has entered an era of rapid development, and T7 RNA Polymerase has been used as a catalytic enzyme in the production process of mRNA vaccines. However, the trace residue of this additive will affect the product quality and its subsequent usage effect. Before the product is put on the market, the residue of additives in its production process needs to be tested to ensure the quality of the product. Therefore, throughout the entire production process, quality control is of vital importance.
However, there are relatively few methods for detecting the residual activity of T7 RNA Polymerase, mainly including radioisotope method, spectrophotometry, fluorescence quenching method, etc. However, the above methods are difficult to achieve high throughput. At the same time, specific oligomeric ribonucleic acid molecules need to be synthesized as substrates, and specific modified RNA probes also need to be designed and synthesized. The cost is relatively high; Either it is prone to radioactive contamination, or it involves many steps and takes a long time.
In the new method for residue detection of T7 RNA Polymerase, the Chinese invention patent CN202110755117.0 discloses a biosensor with signal amplification effect for visual detection of explosive molecules, its preparation method and application. The content of T7 RNA Polymerase is detected by combining T7 RNA Polymerase with a biosensor and converting the chemical signal into an electrical signal through the binding of T7 RNA Polymerase to the receptor. The detection operation is simple and convenient, but the signal of the biosensor is unstable. The differences among various samples are relatively high. Patent US5827661A discloses a novel detection method. By using RNA polymerase for chain amplification, the signal-to-noise ratio during detection can be amplified, resulting in a lower detection limit. However, its detection time cycle is long, the preparation work is complex, and it is greatly affected by the detection environment and personnel operation. The entire material preparation and detection process is complicated, and the error rate is high. The enzyme-linked immunosorbent assay (ELISA) is simple to operate, has a short detection time, can test multiple samples in a short time, and has a relatively high accuracy rate, which greatly facilitates the quality inspection work of mRNA vaccine-related products [1].


At present, Baorui Biotechnology has independently developed a highly sensitive monoclonal antibody against T7 RNA Polymerase (mouse source), which can quickly detect the residue through ELISA with high accuracy, greatly facilitating the quality inspection work of mRNA vaccine-related products. Reagent kits made from self-developed antibodies have been launched on the market.
Product advantages


1. Introduction to Monoclonal Antibodies
monoclonal antibodies (monoclonal antibody) apply lymphocyte hybridoma technology to fuse B lymphocytes and myeloma cells from immunized animals to form hybridoma cells. Through HAT and HT screening, ELISA detection, and limited dilution subcloning, Screen out hybridoma cells derived from a single B cell that have the function of secreting antibodies and can reproduce infinitely. The antibodies produced by these hybridoma cells are monoclonal antibodies [2]. Monoclonal antibodies have high specificity, high uniformity, high efficiency and unlimited supply, and are widely used in immunology, medicine, biology and other fields, including the diagnosis, prevention and treatment of diseases (including cancer).
2. The Development History of monoclonal antibody technology
In the 1970s, B-cell carcinoma multiple myeloma was discovered. It is understood that all these cancerous B cells produce a single type of antibody (a lesion protein), which is used to study the structure of antibodies, but it is still impossible to produce uniform antibodies specific to specific antigens [3].
In 1975, British scientists Milstein and Kohler invented the monoclonal antibody technology. They successfully fused myeloma cell lines with B cells to create hybridomas capable of producing antibodies, for which they were awarded the Nobel Prize in Medicine in 1984.
In 1986, British biochemist Gregory P. Winter and his team pioneered the technology of humanizing monoclonal antibodies [5], eliminating the reactions caused by many monoclonal antibodies in some patients. Therefore, he/she was awarded the Nobel Prize in Chemistry in 2018.
In 2018, American immunologist James P. Allison and Japanese immunologist Tasuku Honjo discovered that by using inhibitory conjugated monoclonal antibodies, negative immune regulation could be suppressed for cancer treatment. Therefore, he/she was awarded the Nobel Prize in Physiology or Medicine. [6]
Monoclonal antibody technology fundamentally resolves the long-standing issues of specificity and repeatability in antibody preparation and can be used to explore:
① The fine structure of proteins;
② Surface neoantigens of lymphocyte subsets;
③ Histocompatibility antigen;
④ Radioimmunoassay (or enzyme immunoassay) analysis of hormones and drugs;
⑤ Localization and classification of tumors;
⑥ Purify microbial and parasitic antigens;
⑦ Immunotherapy and drug-combined immunochemotherapy (" missile "therapy, which uses monoclonal antibodies to specifically bind to target cells and deliver drugs to the lesion site. It can be directly applied to the diagnosis, prevention, treatment of human diseases and the research on immune mechanisms.
For decades, hybridoma technology has matured as a conventional monoclonal antibody preparation technique and has been in use for a long time. Currently, more than 90% of the antibodies approved for therapeutic purposes are produced by this technology.
3. The process of monoclonal antibody preparation (hybridoma technology)

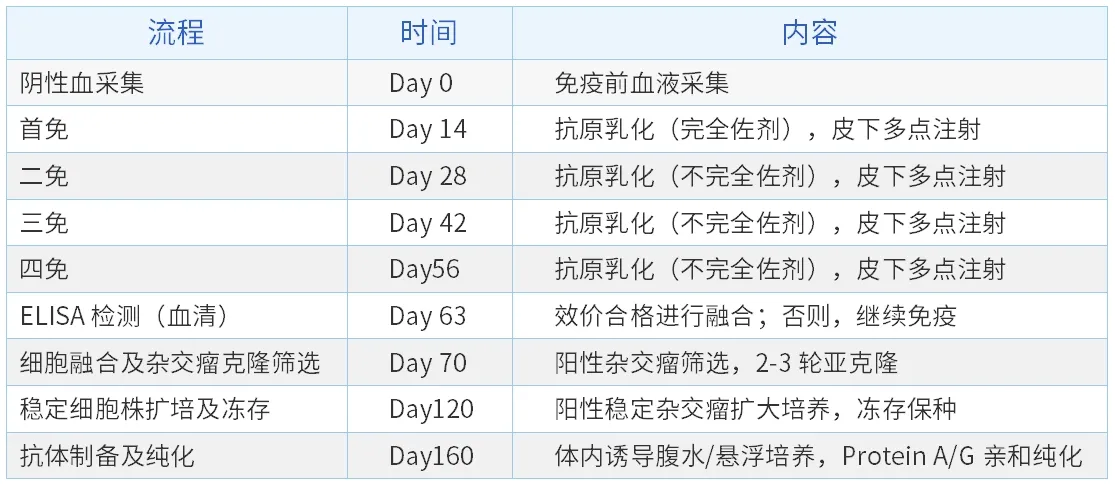
References
[1] Draft Guidelines for Quality Analysis Procedures of mRNA Vaccines. United States Pharmacopeia (USP), 2nd Edition. April 2023.
[2]The preparation process of monoclonal antibodies. Biobang Information. The 2012-05-22.

0756-8699969
Address: No. 88, Shuian 1st Road, Nanping Science and Technology Park, Xiangzhou District, Zhuhai City, Guangdong Province
Email: marketing@biori.com
Wechat official account |
Product consultation |
Join us |
Video Account |






